Areas of Research
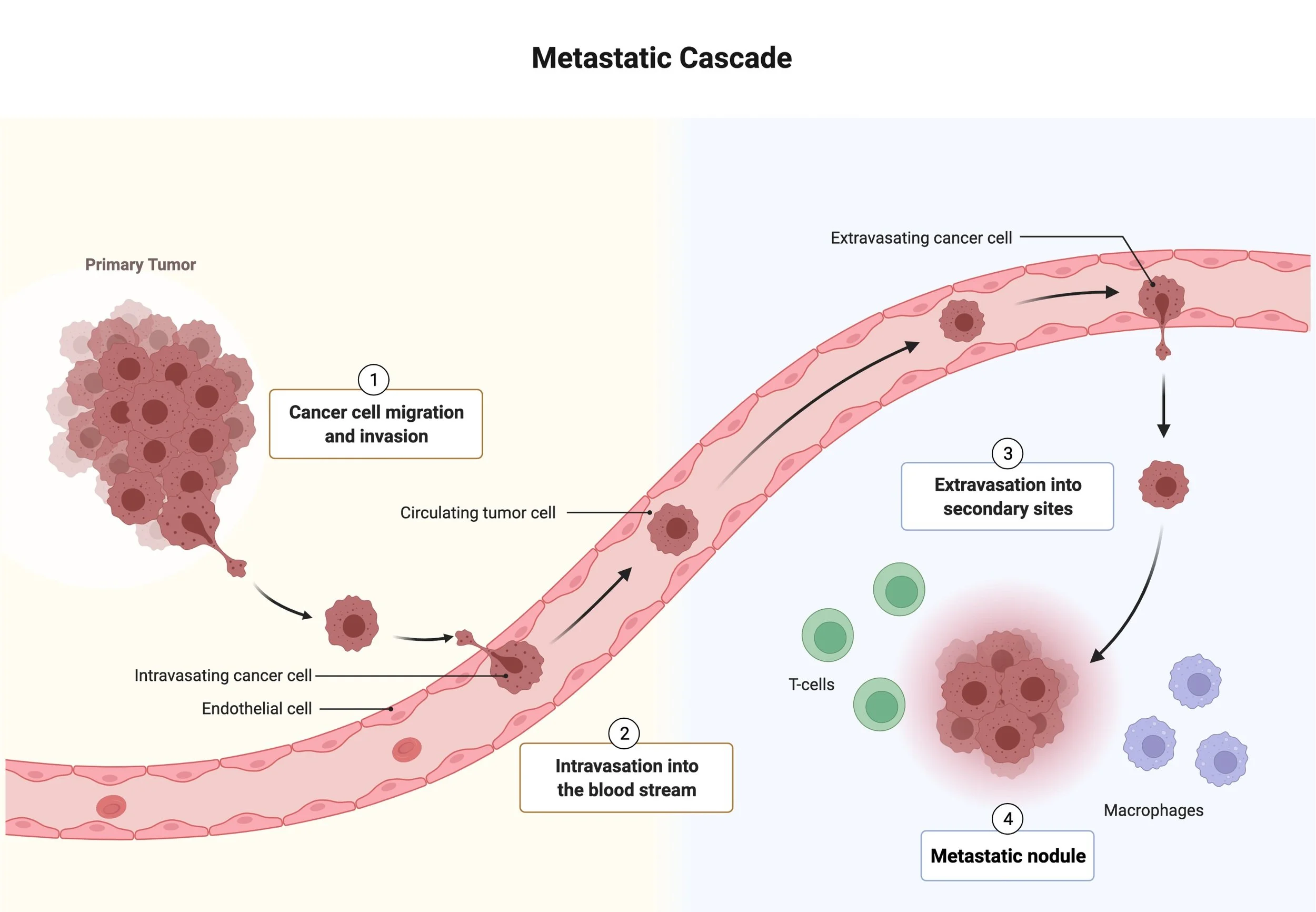
Stress resistance is an integral part of metastasis, as it is a highly inefficient process. Only a few cells are able to survive at each step of the metastatic cascade and colonize vital organs. It has now been recognized that metastasizing cancer cells experience a plethora of stresses imposed by the microenvironment and therapeutic intervention including hypoxia, nutrient limitation, oxidative stress and more, yet they are able to overcome them by relying on specific stress-resistance mechanisms. We have previously showed that high levels of oxidative stress limit survival of metastasizing cancer cells in circulation and metastatic spread into the organs (Piskounova et al, Nature, 2015). This discovery has now become a major focus of metastasis research and has now been observed across many other cancer types. To survive under oxidative stress, cancer cells have to rewire their metabolic pathways to up-regulate their antioxidant capacity. Once metastasizing cells extravasate, they have to adapt further to the different metabolic milieu of the target organs, as well as perfusion- and diffusion-limited hypoxia due to lack of vasculature and oxygen gradients that exist at the target site. Lack of vasculature also puts disseminated cancer cells under nutrient stress. Since different organs represent different and specialized environments, cancer cells have to adjust to alternative fuels available in each organ, such as glucose in the liver, acetate in the brain and lipids in the omentum, in case of ovarian cancer. It is therefore strikingly clear that successful metastasizers have to implement a multitude of stress-resistance mechanisms to overcome many of these barriers in order to progress to overt metastatic disease. Identification of key regulatory hubs that confer stress resistance on metastasizing cells is a lucrative and potentially effective strategy to pinpoint therapeutic targets specific to metastatic disease.
Ongoing Projects
-

Metabolic Adaptations
Disseminating cancer cells have to rewire their metabolic pathways to up-regulate their anti-oxidant response. Key metabolic pathways can be quickly altered to rapidly respond to their changing microenvironment. After extravasation, cells must adapt to the different metabolic milieu of each colonized organ, oxygen and nutrient availability.
-
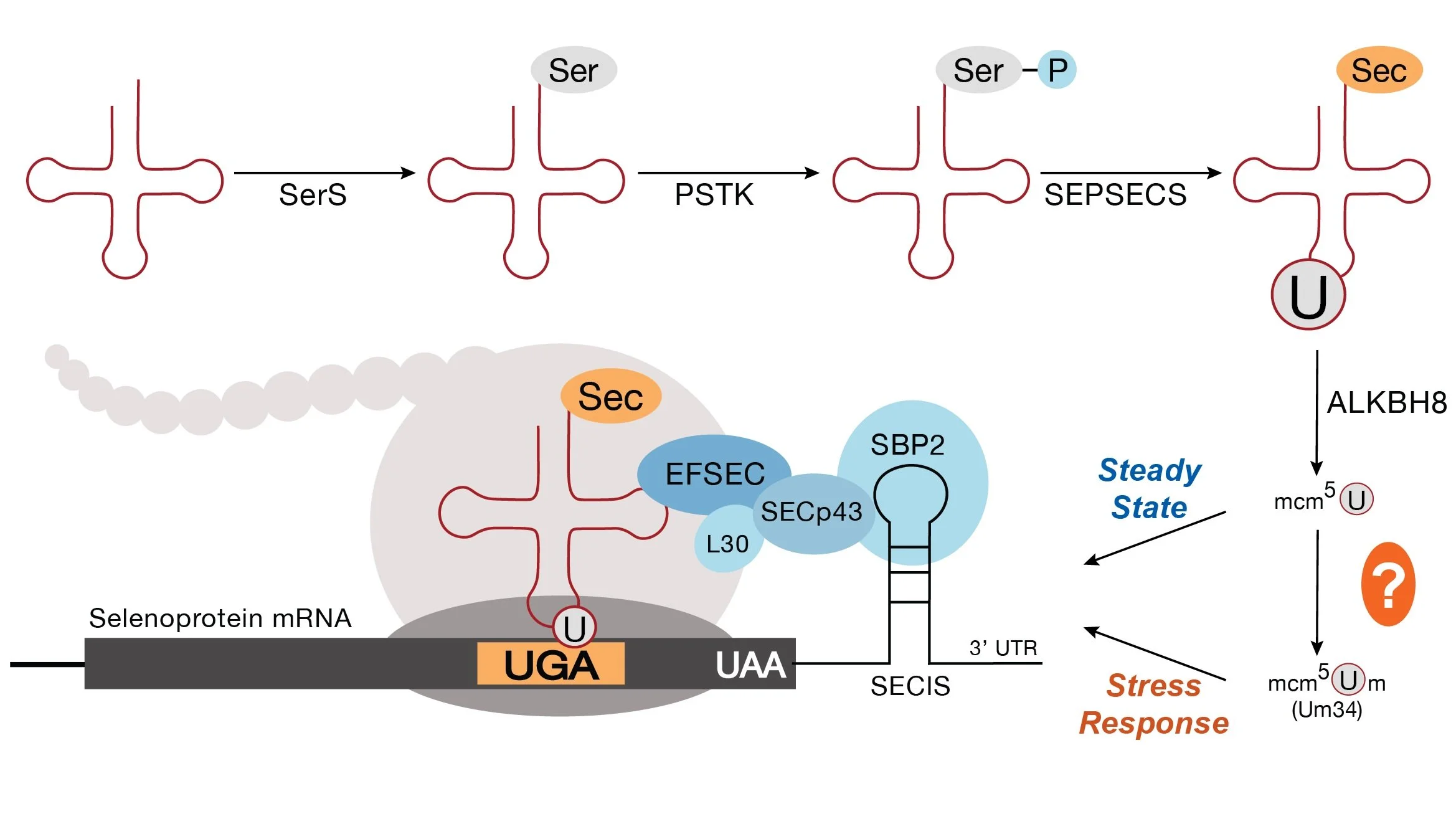
Selenocysteine Proteome
The 21st amino acid is coded for by a UGA stop codon, and typically occurs in specialized RedOx balancing enzymes such as GPX1, GPX4 , and TXNRD1 due to its high pKa relative to cysteine. Our lab is interested in understanding the regulation of the selenoproteome at the translational level in metastasis.
-
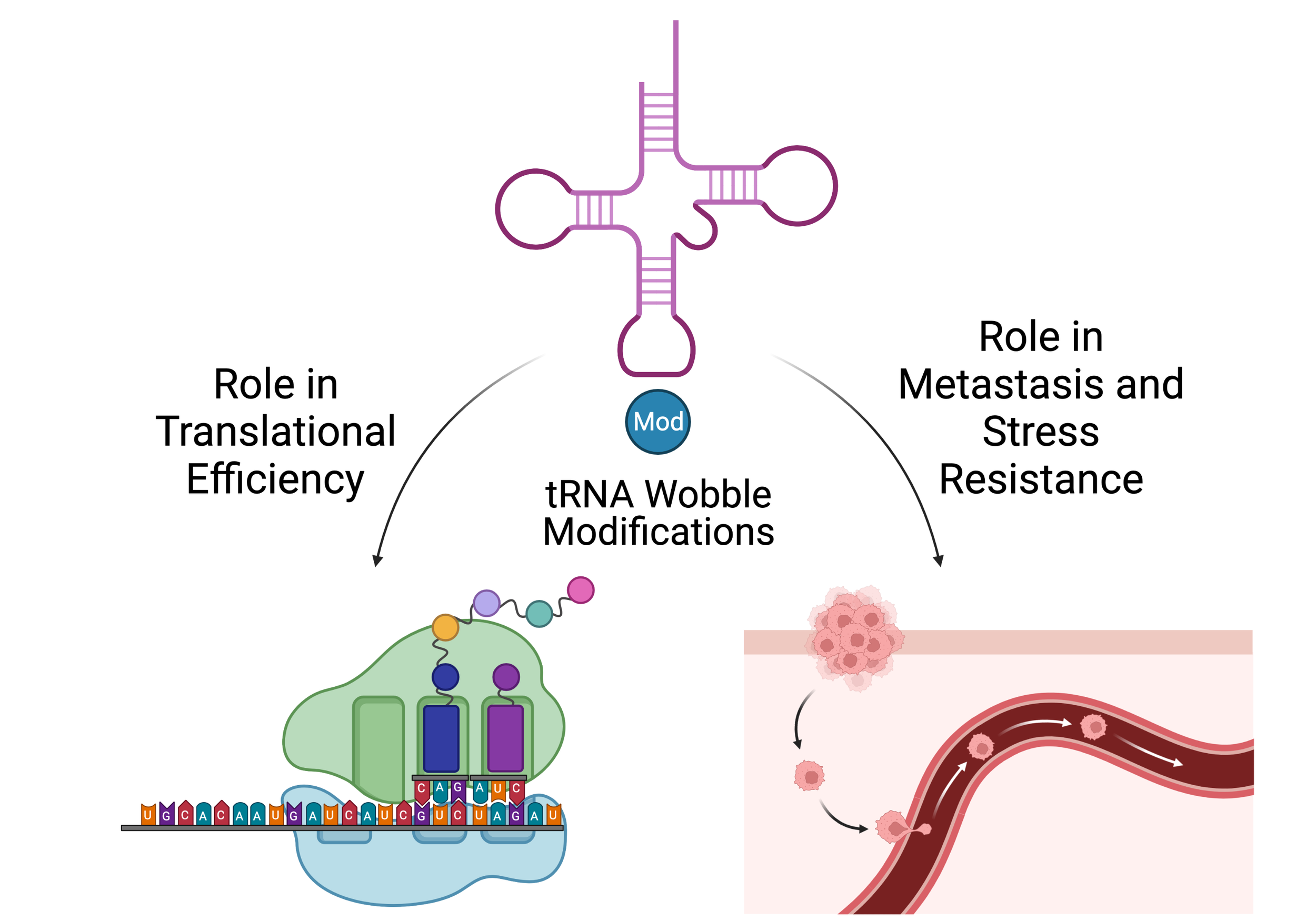
tRNA Wobble Modifications
Metastasizing cells must rapidly adapt to to their changing microenvironment. Our lab is interested in understanding the regulation of the stress response proteome at the translational level through tRNA modifications and codon-biased translation.